Which Organic Compounds Are Always Insoluble In Water
The factors that influence the relative boiling points and water solubilities of various types of compounds were discussed earlier. Some of us drink it straight from our faucet without a second thought.
Spectator Ions Are Those Which Are In Solution But Do Not React Are There Any Spectator Ions For The Reaction Of Sodium Phosphate With Calcium Bromide Socratic
Safe water isnt straightforward but the best-for-you best-for-the-planet solution is.

Which organic compounds are always insoluble in water. Keep in mind however that there are exceptions to every category in this table. Only 5-10 of the nonvolatile organic compounds that comprise the remaining 90 of the total organic matter have been identified. Soluble and insoluble fibres make up the two basic categories of dietary fibre.
In dry lands covering over 65 cultivated area in India application of chemical fertilizers and pesticides is always low. Dietary fibre although not always defined as such has been consumed for centuries and is recognized for having health benefits. Despite such figures the 2 oxidation state is rare with 4 and 6 being more common.
The physical properties of alkanes reflect the fact that alkane molecules are nonpolar. So these areas are at least relatively organic or organic by default and a portion of these lands can be converted easily to an organic one to provide better yieldsreturns. Others go to great lengths to buy enough jugs or bottles from the store to always have on hand.
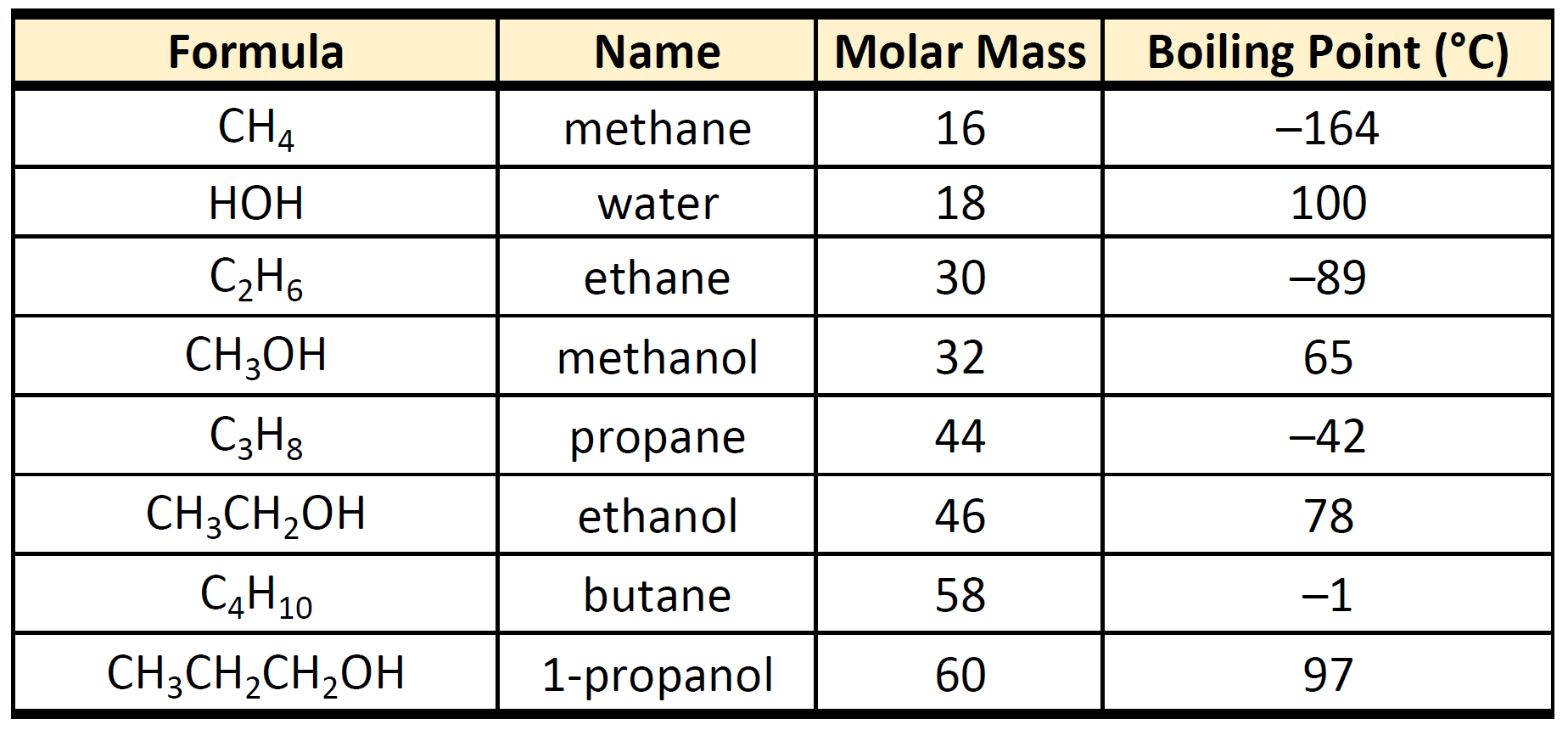
Soluble in water than compounds of similar MW since they can hydrogen bond to more than one water molecule. The EPA 1978c has categorized the organic compounds in drinking water into five different classes. On the other hand most organic compounds have covalent bonds between molecules and hence are insoluble in water though they are soluble in other organic solvents.
However theres reason to eat cucumbers all year long. For condensed phases solids and liquids the pressure dependence of solubility is typically weak and usually. In compounds magnesium virtually always exhibits a 2 oxidation state because of the loss or sharing of its two 3s electrons.
There are however a small number of coordination compounds known with magnesium-magnesium bonds LMgMgL in which the magnesium centres have a formal 1 oxidation state. The first and second ionization energies of sulfur are 9996 and 2252 kJmol respectively. An alkyl group is a unit formed by removing one hydrogen atom from an alkane.
Cellulose hemicellulose and lignin- are not soluble in water whereas pectins gums and mucilages- become gummy in water. Ethers are essentially non-polar and insoluble in water. With vitamin K B vitamins copper potassium vitamin C and manganese.
Again changes in crystal packing and intermolecular forces are responsible. Most macromolecules are polymers molecules that consist of a single unit monomer repeated many times. Thus palmitoleic acid melts over 60º lower than palmitic acid and similar decreases occur for the C 18 and C 20 compounds.
Each class has distinctly different characteristics of concern to those involved in water treatment. Like watermelon cucumbers are made up of mostly 95 percent water which means eating them on a hot summer day can help you stay hydrated. Many carboxylic acids that are liquids at room.
Organic compounds are those that have carbon atomsIn living systems large organic molecules called macromolecules can consist of hundreds or thousands of atoms. Alkanes alkenes and alkynes are essentially non-polar and insoluble in water. Alkanes are insoluble in water and less dense than water.
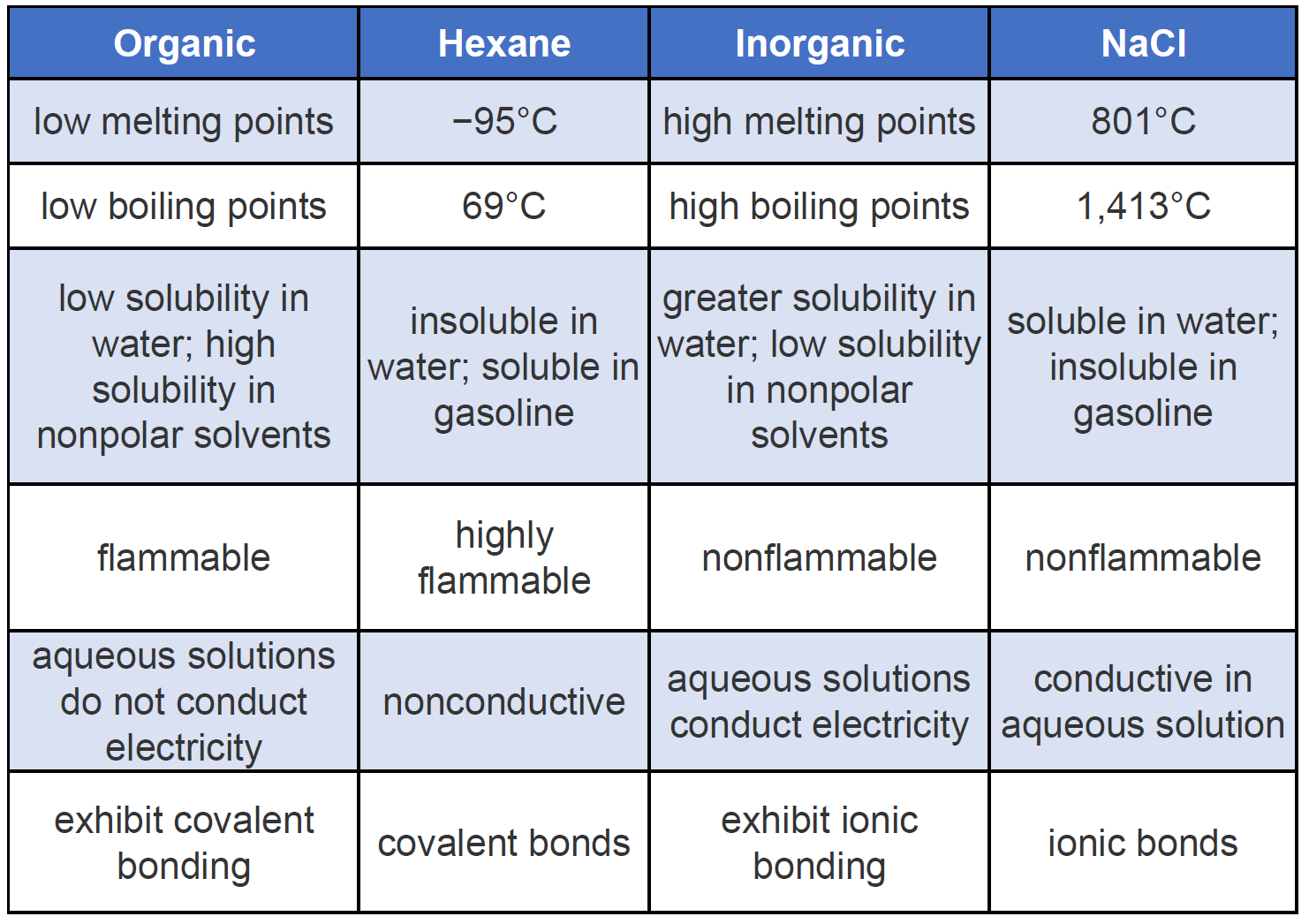
Sulfur is insoluble in water but soluble in carbon disulfide and to a lesser extent in other nonpolar organic solvents such as benzene and toluene. To further illustrate typical differences among organic and inorganic compounds Table PageIndex1 also lists properties of the inorganic compound sodium chloride common table salt NaCl and the organic compound hexane C 6 H 14 a solvent that is used to extract soybean oil from soybeans among. Cucumbers belong to the same plant family as squash pumpkin and watermelon the Cucurbitaceae family.
The IUPAC System of Nomenclature provides rules for naming organic compounds. Organic compounds are formed as a result of actions of living organisms while inorganic compounds are formed due to natural processes unrelated to any life form or as a result of. There is very little intermolecular association because the carbon-hydrogen bond is non-polar.
The solubility of organic compounds nearly always increases with temperature. 16 Physical Properties of Carboxylic Acids As the number of carbons in a carboxylic acid series becomes greater the boiling point increases and the solubility in water decreases. The technique of recrystallization used for purification of solids depends on a solutes different solubilities in hot and cold solventA few exceptions exist such as certain cyclodextrins.

Chemistry Ii Water And Organic Molecules
Solubility And Dissolving Vce Chemistry

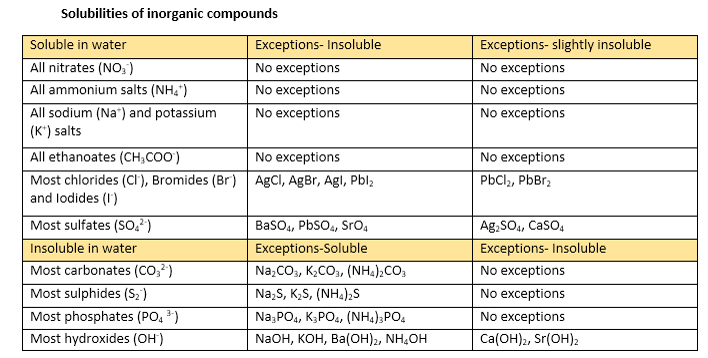
Soluble And Insoluble Compounds Chart Solubility Rules Table List Of Salts Substances Youtube
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry

Which Compound Is The Most Soluble In Wate Clutch Prep
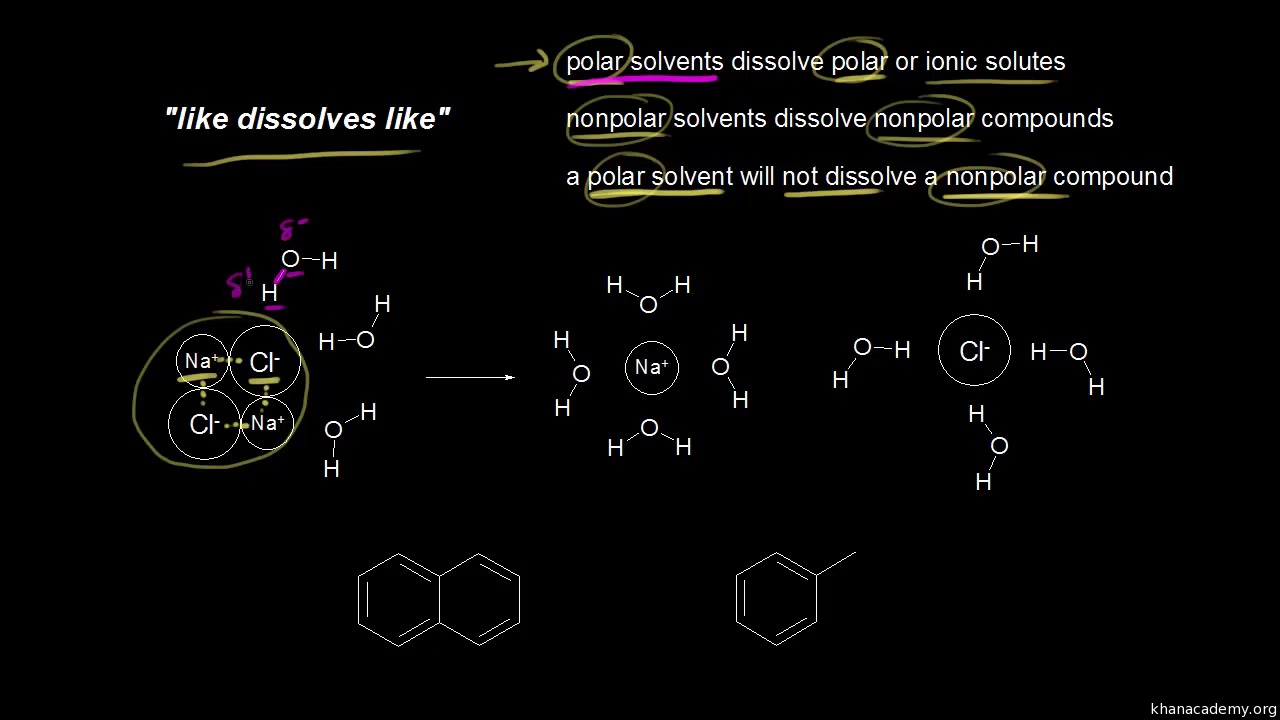
Solubility Of Organic Compounds Video Khan Academy

Solubility Introduction To Chemistry

Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry

Solubility Of Organic Compounds Video Khan Academy
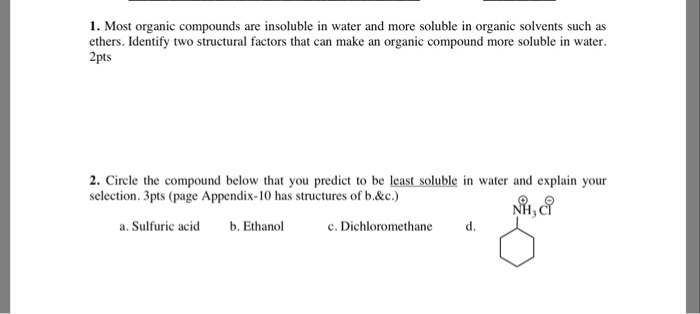
Solved 1 Most Organic Compounds Are Insoluble In Water And Chegg Com
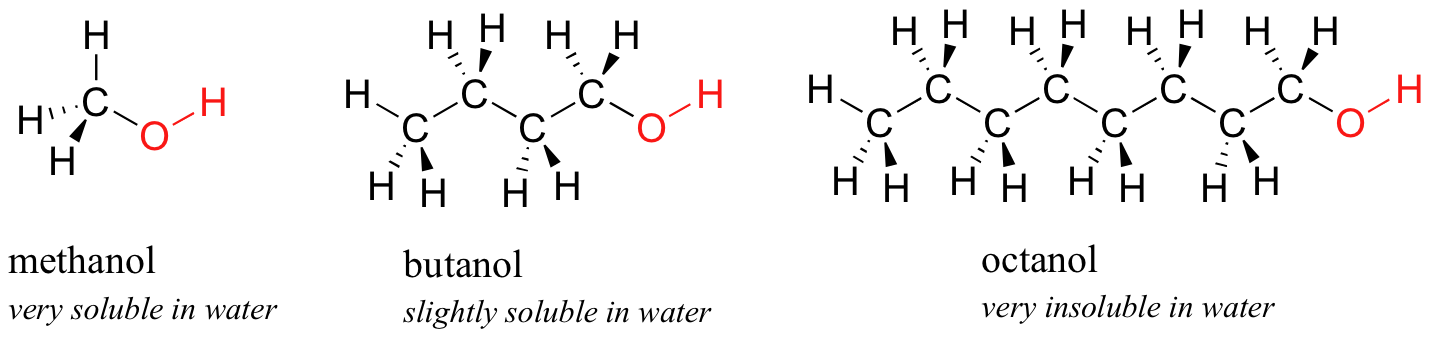
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts

Solved Why Are Most Organic Compounds Nonconducting And Chegg Com
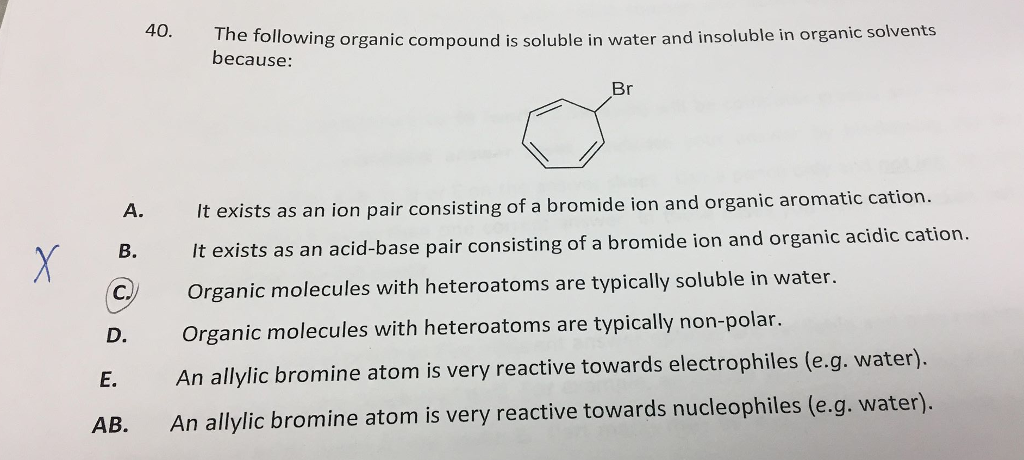
Solved The Following Organic Compound Is Soluble In Water Chegg Com
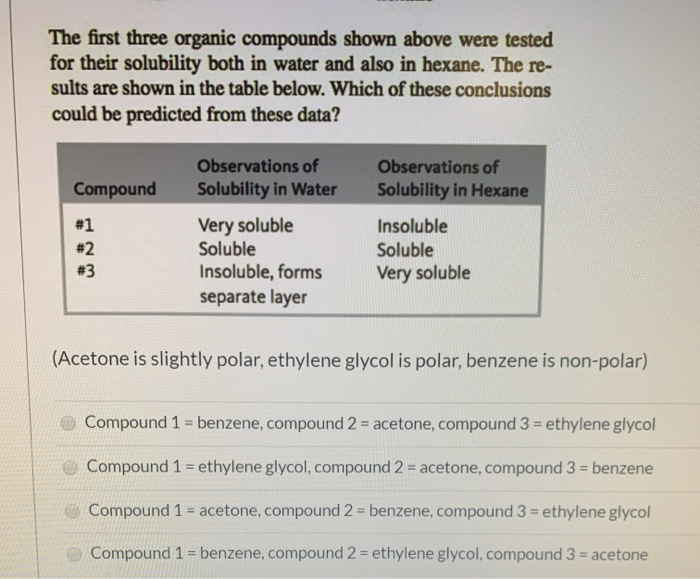
Solved The First Three Organic Compounds Shown Above Were Chegg Com
Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry

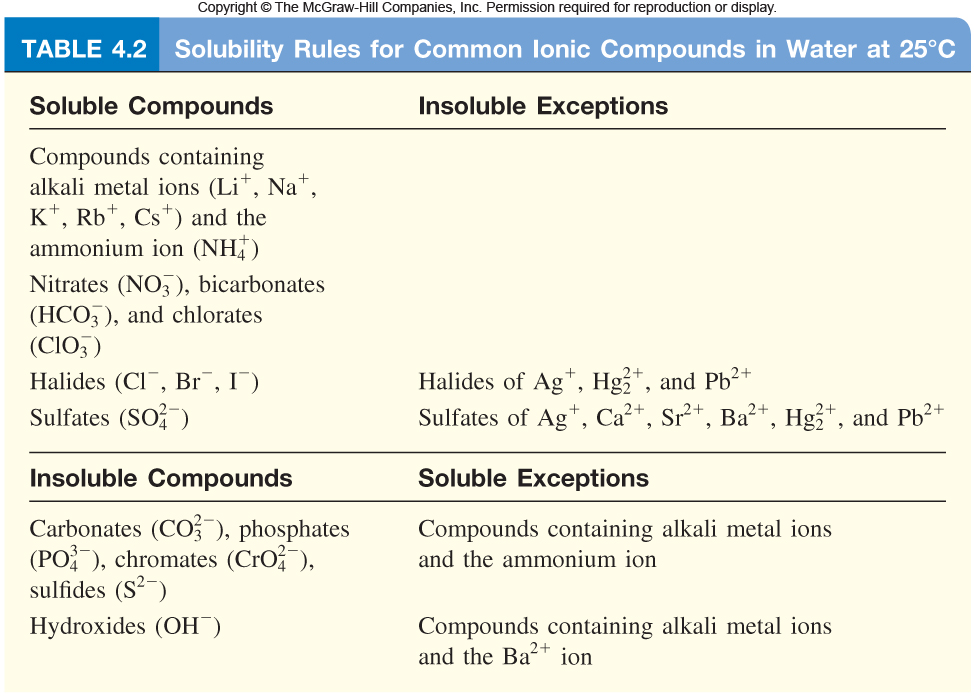
Solubility Rules Chart Chemistry Chemtalk
Which Are Soluble In Water Covalent Compounds Or Ionic Compounds Quora

Solubility In Water For The Compounds Discussed In This Work Download Table
Is Hcl Hydrochloric Acid Soluble Or Insoluble In Water Youtube